Opioids
sometimes called narcotics, are a group of drugs that act on the central nervous system to produce morphine-like effects such as pain relief and euphoria.
Content:
1. Neurotransmission of pain signal
2. Endogenous opioids & opioid receptors
3. Buprenorphine
4. Naloxone
5. Reference
2. Endogenous opioids & opioid receptors
3. Buprenorphine
4. Naloxone
5. Reference
1. Neurotransmission of pain signal
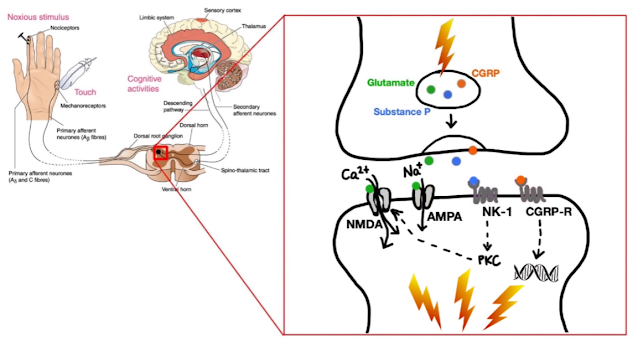
Pain begins at the nociceptors
- high threshold primary sensory neurons respond to damage to the body by transmitting the painful stimulus to the second-order neurons in the dorsal horn of the spinal cord.
- signal is carried through
- spinothalamic tract
- thalamus
- somatosensory cortex where pain is perceived
On a microscopic level,
- pain signal takes the form of a series of action potentials
- fire repeatedly depending on the intensity of pain
- To enhance movement across the synaptic cleft, transmitter chemicals are released from the presynaptic neurons, including
- glutamate
- activate both NMDA and AMPA receptors
- permit influx of positively charged calcium and sodium ions respectively
- makes the neuron more likely to fire.
- In this way glutamate excites the second-order neurons in the dorsal horn, which leads to propagation of a sharp, localized pain signal.
- substance P
- binds to the neurokinin-1 (NK-1)
- leads to intracellular signaling that involves activation of
- arachidonic acid pathways
- nitric oxide synthesis
- NMDA receptors
- NMDA receptors are activated when Substance P attaches to NK-1 receptors
- then gets incorporated into the cell, activating Protein Kinase-C.
- removes the magnesium (normal conditions: blocking NMDA receptor)
- allows glutamate to attach to the NMDA receptor
- permit the inflow of calcium ions
- causing the pain signal to increase and fire more frequently.
- calcitonin gene-related peptide (CGRP)
- binds to its receptor on second order neurons
- changes in receptor expression and function
- altered neuronal activity
- contributes to central sensitization
- characterized by lowered threshold for evoking action potentials
2. Endogenous opioids & opioid receptors
- Our bodies can cope with certain amount of pain by releasing endogenous opioids.
- Three major families of endogenous opioids:
- enkephalins
- dynorphins
- endorphins
- Endogenous opioids exert their effects by binding to opioid receptors which are abundantly present in the central and peripheral nervous systems.
- Three major types of opioid receptors
- µ (mu)
- δ (delta)
- k (kappa)
- All opioid receptors are 7-transmembrane spanning proteins that couple to inhibitory G-proteins
- all present in high concentrations in the dorsal horn of the spinal cord.
- Activation of these receptors by an agonist, such as the endogenous μ-opioid peptide endorphin
- closing of the voltage-gated calcium channels on the presynaptic nerve terminals
- decreases the release of neurotransmitters, such as glutamate, substance P and calcitonin-gene-related-peptide.
- Activation of opioid receptors
- opening of potassium channels
- allowing efflux of potassium ions
- hyperpolarization, rendering neurons less sensitive to excitatory inputs.
- the majority of currently available opioid analgesics act primarily at the μ-opioid
- essentially mimicking the effects of endogenous opioid peptides
- However, while naturally-derived opioids can only reach a certain potency, the synthetically-produced opioids are refined and processed to be much more powerful.
- synthetic opioid agonists:
- Fentanyl
- Hydrocodone
- Hydromorphone
- Methadone
- Meperidine
- Oxycodone
- Oxymorphone
**Methadone
- a potent μ-receptor agonist
- potent antagonist of the NMDA receptor
- norepinephrine and serotonin reuptake inhibitor
- useful for treatment of both nociceptive and neuropathic pain.
Side effects
All opioids
- produce some degree of nausea
- due to direct stimulation of the chemoreceptor trigger zone in the medulla
- produce a dose-dependent respiratory depression
- by reducing brain stem respiratory center responsiveness to carbon dioxide
- depress the respiratory centers in the pons and medulla, which are involved in regulating respiratory rhythmicity
- Antitussive effect by depressing the cough center in the medulla
- suppression of the immune system
- as opioid receptors are involved with regulation of immunity.
- produce a dose-dependent bradycardia
- by increasing the centrally mediated vagal stimulation.
- itching
- via central action on pruritoceptive neural circuits
- constipation
- decrease gastric motility and prolong gastric emptying time
- antidiuretic
- depress renal function
- urinary retention
- increase sphincter tone
- addiction
- by causing both physical and psychological dependence
- euphoric effect
- involve GABA-inhibitory interneurons of the ventral tegmental area of the brain
- when opioids attach to and activate the µ receptors in that area
- the release of GABA becomes suppressed
- increases dopamine activity
- increases the amount of pleasure felt.
- withdrawal symptoms
- prolonged, regular use of opioids
- leads to desensitization of receptor signaling & down-regulation of the receptors
- decrease in sensitivity to the effects of opioids
- These symptoms generally are opposite to the pharmacological effects of the opioid drugs.
- rather than causing constipation and slowing respiration, the brain stem triggers diarrhea and elevates blood pressure.
- Instead of triggering happiness, the nucleus accumbens and amygdala reinforce feelings of dysphoria and anxiety.
- negativity feeds into the prefrontal cortex, further pushing a desire for opioids
Morphine & Meperidine
- hypotension
- provoke release of histamine
- flushing of skin of the face, neck, and upper thorax
- dilation of cutaneous blood vessels
Meperidine
- tachycardia
- its structural similarity to Atropine
3. Buprenorphine
- partial µ receptor agonist
- binds to the receptor and activates it with a smaller shape change which leads to only a partial receptor response.
- the effects of partial agonists increase only until they reach a plateau
- an antagonist at the δ and κ receptors---mixed agonist-antagonist
- contributions of these actions to its analgesic profile are currently unclear
- Side effect: respiratory depression & euphoria
- Benefits: lower risk of abuse, addiction, and side effects.
4. Naloxone
- opioid antagonist
- block or reverse the effects of opioid drugs
- works by knocking off the opioids attached to the receptors in the brain
- thereby temporarily stopping the opioid effect
- possible because Naloxone has a stronger affinity for opioid receptors
- able to kick the opioids out and block them from attaching again
- Use: during an emergency situation when a person’s breathing has slowed down or stopped due to an opioid overdose, Naloxone can quickly restore normal breathing and save the life.
5. Reference
https://youtu.be/t2tKyjj7u5Y
NEXT:
PREVIOUS:





Comments
Post a Comment