Pharmacokinetics
1. Definition
Pharmacokinetics is currently defined as the study of the time course of drug absorption, distribution, metabolism, and excretion.
Clinical pharmacokinetics is the application of pharmacokinetic principles to the safe and effective therapeutic management of drugs in an individual patient.
2. Step
a. Absorption: Movement of a drug from its site of administration to the bloodstream
Happen in 4 different ways
- Passive diffusion
- most of drug
- simply move from area of high concentration to area of lower concentration
- water-soluble: move through a channel or pore
- lipid-soluble: pass through membrane
- Facilitated diffusion
- large molecule
- move from area of high concentration to area of lower concentration through a carrier protein
- Active transport
- require energy from hydrolysis of ATP to ADP (breaking of phosphate bond)
- Endocytosis
- large molecule
- engulfment by cell membrane
*Absorption is a variable process depending on pH surface area and blood. 👉Bioavailability
When a drug is given intravenously, absorption is not required, and bioavailability is 100% because the active form of the medicine is delivered immediately to the systemic circulation.
However, orally administered medications have incomplete absorption and result in less drug delivery to the site of action. For example, many orally administered drugs are metabolized within the gut wall or the liver before reaching the systemic circulation. This is referred to as first-pass metabolism, which reduces drug absorption.
So, the bioavailability is 👇
b. Distribution: Journey of the drug through the bloodstream to various tissues of the body
Dependent on factors:
- Lipophilicity
- Lipophilic drug ↑ (easy to dissolve through some membrane)
- Hydrophlic drug ↓
- Blood flow
- Brain ↑ (high blood flow, accumulate faster)
- Skin ↓
- Capillary permebility
- Liver ↑ (lots of slit junctions through which large proteins can pass)
- Brain ↓
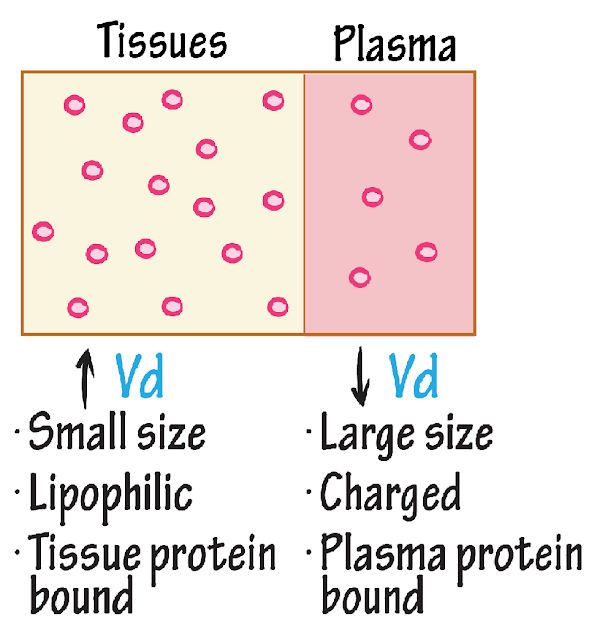
- Plasma & Tissue binding
- Albumin ↓ (Binding with major drug binding protein, slow down the process)
- Volume of distribution
- Lower molecular weight drugs ↑
- High molecular weight drugs ↓
- Formula:
c. Metabolism: process that breaks down the drug.
- mainly through hepatic renal and biliary route
- total body clearance is simply the sum of individual clearance processes
- First order kinetics & Zero order kinetics & Steady state
First order kinetics
- Most of drug
- the amount of drug eliminated over time is directly proportional to the concentration of drug in the body.
- eg. starting with 1000 milligrams of a drug the amount eliminated per each time period will be different but the fraction will be constant, which is 16%
Zero order kinetics
- Few drugs such as Aspirin
- the amount of drug eliminated is independent of drug concentration in the body, so the rate of elimination is constant
- eg. starting with 1000 milligrams of a drug the amount eliminated per each time period will be same, which is 200 milligrams, but the fraction will be different
- easy to determine half-life of a drug
- the time that is required to reduce drug concentration in plasma by a half
- helps to predict steady state concentrations
Steady state
when doses of a drug are repeatedly administered, a drug will accumulate in the body until the rate of administration equals the rate of elimination
- Graph it when after each additional dose the peak and trough concentrations stay the same
- typically attained in about 4 to 5 half-lives
- concentration of a drug high enough to be effective but not too high to be toxic so the goal is to maintain steady state concentration within therapeutic range
- Large loading dose: when life-threatening infections
- to reach desired concentration more rapidly
d. Excretion: the removal of the drug from the body
- through kidney which excrete drugs into the urine
- however a kidney can't efficiently get rid of lipid soluble drugs as there a passively reabsorbed
- Liver comes to the rescue by transforming lipophilic drugs into water soluble substances that are then easily removed by kidneys
- mainly through two metabolic reactions: phase 1 and phase 2
Phase 1--Oxidation, Hydrolysis, Reduction
- making a drug more hydrophilic
- these reactions involve introduction or unmasking of a polar functional group
- catalyzed by cytochrome p450 enzymes
Still too lipophilic👇
Phase 2--Glutathione conjugation, Acetylation, Sulfation and Glucuronidation
- conjugation reaction which involves addition of a polar group
- these reactions produce polar conjugates which cannot diffuse across membranes, therefore they're easily eliminated from the body
Cytochrome p450: Large family of enzymes is essential for the metabolism of drugs
Catalyze vast majority of phase 1 reactions:
- CYP 3A4
- CYP 3A5
- CYP 2D6
- CYP 2C8
- CYP 2C9
- CYP 1A2
Drug interactions arise from drug's ability to induce or inhibit,
Some of the important inducers (PCRABS)
- Phenytoin
- Carbamazepine
- Rifampin
- Alcohol (chronic)
- Barbiturates
- St. John's Wort
Some of the important inhibitors (GPACMAN)
- Grapefruit
- Protease inhibitors
- Azole antifungals
- Cimetidine
- Macrolides (except Azithromycin)
- Amiodarone
- non-dihydropyridine calcium (Diltiazem + Verapamil)
3. Reference:
https://drawittoknowit.com/course/pharmacology/glossary/pharmacology/volume-of-distribution
https://www.youtube.com/watch?v=NKV5iaUVBUI







Comments
Post a Comment